Shenzhen Cell Valley and HUST Shenzhen Hospital released a case report on CAR-T treatment of lymphoma
introduction
On May 4, 2024, Professor Guo Zhi's team from the Department of Hematology of Union Shenzhen Hospital of Huazhong University of Science and Technology and Professor Wang Jianxun's team from Shenzhen Cell Valley published a report in the journal International Immunopharmacology (International ImmunoPharmacology, District 2, Chinese Academy of Sciences). IF 5.6) "Rapid response in relapsed follicular lymphoma to novel anti-CD19 CAR-T therapy with Novel anti-CD19 CAR-T Therapy" was jointly published in SCI journal pseudo-progression and cytomegalovirus infection A case report ". The co-corresponding authors of this case report are Professor Guo Zhi, Deputy chief physician Liu Liqiong, and Professor Wang Jianxun, chief scientist of Shenzhen Cell Valley, Department of Hematology, Union Shenzhen Hospital, Huazhong University of Science and Technology. The co-first authors are attending physician Zhong Nan, assistant researcher Ma Qihong, and assistant researcher Gong Shiting.
This case shows that the novel anti-CD19 CAR-T produced by Shenzhen Cell Valley successfully treated a patient with recurrent follicular lymphoma complicated with cytomegalovirus infection. The end-stage lymphoma patient was in complete remission after CAR T infusion, and has not recurred for 7 months so far, which is recognized as clinically cured.
This case demonstrates that the novel CD19-targeting CAR T cells prepared by Shenzhen Cell Valley using retroviral vectors have good efficacy in patients with drug-resistant refractory follicular lymphoma, and the safety, effectiveness and therapeutic response are all in line with expectations.
Shenzhen Cell Valley is committed to enabling research and development, improving the speed of clinical transformation, reducing costs for industrialization, and seeking new life for patients, so that good products can go out of the laboratory, truly research and development to serve the clinic, and be applied to the clinic. At present, Shenzhen Cell Valley has worked closely with the Hematology Department of Shenzhen Union Hospital, Huazhong University of Science and Technology, and has conducted clinical treatment of several novel CAR T cells targeting CD19, BCMA, CD19/CD22, CD7, CD38 and other novel CAR T cells prepared based on retrovirus vectors, all of which have shown good effectiveness and safety. In the future, the two parties will further cooperate in clinical research of CAR-T and other cellular immunotherapy products.
Shenzhen Cell Valley provides GMP grade retroviral vectors and cell therapy products with high safety, good quality and low price to ensure the safety and effectiveness of cell therapy products and promote the overall development of cell and gene therapy industry.

Figure 1. Case report paper information
(DOI: 10.1016/j.intimp.2024.112174)
Case report presentation
In this paper, a case report was selected to briefly introduce the course of the novel anti-CD19 CAR-T treatment for patients with recurrent follicular lymphoma complicated with cytomegalovirus infection.
A 40-year-old male patient had no obvious inducement in early May 2021, a palpable mass in the lower abdomen of the xiphoid process, no pain, no nausea, vomiting, weight loss and other discomfort. The patient was diagnosed with lymphoma after CT, tumor puncture, immunohistochemistry and other examinations (Figure 2). The tumors of the lymphostomy system were mainly follicular structures, and follicular B-cell lymphoma was considered as grade 2 and stage 3, with FLIPI score of 2. After several rounds of chemotherapy, the patient's condition improved slightly. On September 5, 2023, the patient's re-examination of PET-CT(Figure 2B-b) indicated that the tumor was in a state of inhibition and partial remission (PR) after treatment, but some lesions still remained, and the attending physician decided to perform CAR-T therapy on the patient.
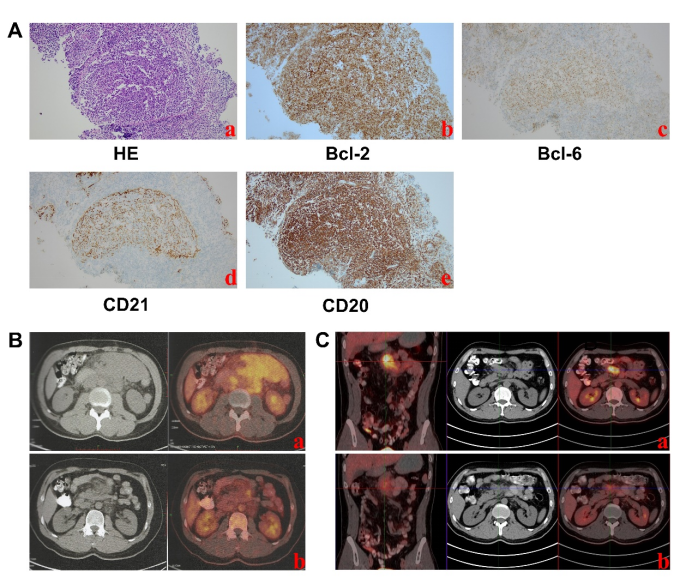 Figure 2. PET-CT images and pathological sections before CAR-T infusion. (A) Pathological findings of abdominal mass before CAR-T infusion. a: Hematoxylin eosin staining. b-e: Positive for Bcl-2, positive for Bcl-6, positive for CD21, positive for CD20. (B) PET-CT imaging time for initial diagnosis and effective treatment of the abdomen. a:2021-5-26. b: 2021-12-14. (C) PET-CT time and immunomaintenance therapy for recurrent abdomen. a:2023-2-16. b: 2023-9-5.
Figure 2. PET-CT images and pathological sections before CAR-T infusion. (A) Pathological findings of abdominal mass before CAR-T infusion. a: Hematoxylin eosin staining. b-e: Positive for Bcl-2, positive for Bcl-6, positive for CD21, positive for CD20. (B) PET-CT imaging time for initial diagnosis and effective treatment of the abdomen. a:2021-5-26. b: 2021-12-14. (C) PET-CT time and immunomaintenance therapy for recurrent abdomen. a:2023-2-16. b: 2023-9-5.
With excellent product quality, solid and efficient service style, top technical team and high-quality corporate reputation, Shenzhen Cell Valley successfully won the bid for the "GMP grade CAR T cell preparation" project of Huazhong University of Science and Technology Union Shenzhen Hospital. Therefore, in this case, Shenzhen Cell Valley provided retrovirus-based retroviral vector to prepare GMP-grade new targeted CD19 CAR-T cells. The positive rate of the GMP-grade CAR-T cells was 52.9%, and the purity reached 100%, which strictly met the release standards (FIG. 3A, 3C).

Figure 3. Construction of CD19 CAR T cells and infusion scheme of CD19 CAR T cells. (A) Schematic diagram of CD19 CAR vector. (B) CAR T infusion protocol, FC for lymphocyte depletion CAR T cells at a dose of 3×106/kg. (C) CD19 CAR T cell product quality.
Lymphocyte collection was performed 14 days before infusion, and 50 ml of CD19 CAR T cells (3×106/kg) were transfused 5 days after lymphocyte removal by fludarabine and cyclophosphamide pretreatment (FIG. 3B). Two days after CAR T infusion, the patient developed grade 2 CRS, bilateral subcervical lymph node enlargement, a sharp increase in IL-6, high fever (39.8°C), and cytomegalovirus infection was subsequently detected. However, after symptomatic treatment, the indicators of the patient returned to normal level. After being uated by the clinician, the patient can be discharged to recuperate at home and be reviewed regularly.
In addition, we measured the proportion of CD4+/CD8+T cells and found that it increased after a decline on day 60, while the number of CAR copies increased first and then decreased. We speculate that the tumor may have recurred around day 60, dormant CAR T cells are activated, resulting in elevated CAR copy numbers, cytotoxic CD8+ T cells proliferate and kill the tumor, and PET-CT at month four (Figure 5) suggests that the patient has achieved complete response (CR).
The results demonstrated the safety, efficacy and therapeutic response of CD19-targeting CAR T cell therapy prepared by retroviral vector in Shenzhencell were in line with expectations. After long-term follow-up, the CAR gene could still be detected in peripheral blood 7 months after infusion. At this time, the targeted CAR T cells are mostly of memory phenotype. Once the tumor has a recurrence trend, the CAR T cells can still be activated to monitor and permanently remove the tumor cells at any time to avoid tumor progression.
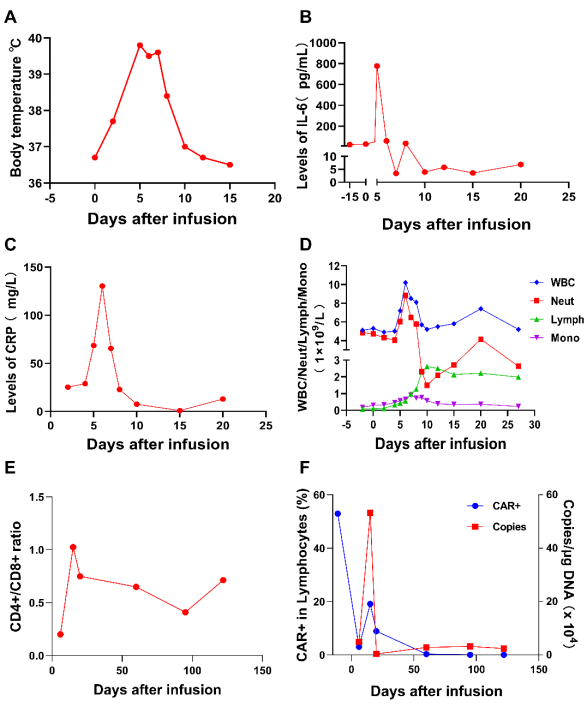
Figure 4. Clinical response and key indicators of CD19 CAR T cell infusion. (A) Changes in patient body temperature after CAR-T infusion. On day 5, the body temperature rose to 39.8℃, and on day 8, the body temperature began to drop. (B) IL-6 levels after CAR T cell infusion. IL-6 reached its peak on day 5 and returned to normal on day 10. (C) Changes in patients' CRP. CRP reached its highest value within one week after infusion (130.32 mg/L). (D) Changes in WBC, Neut, Lymph and Mono; (E) Changes in CD4+/CD8+ ratio in CD3+ T cells; (E) The level of CAR-t cell expansion measured by flow cytometry and the number of CAR-DNA copies measured by qPCR. CAR-T and CAR-DNA copy number fluctuated after infusion and peaked at day 15 (CAR-T in CD3+ T cells :19.1%, CAR-DNA copy number :532350 copies /μg).
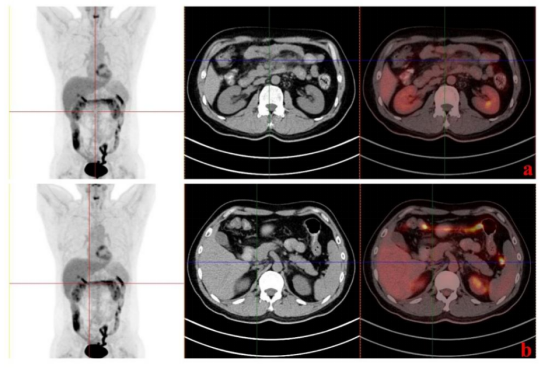
Figure 5. PET-CT examination 4 months after infusion



